Abstract
Children with acute myeloid leukemia (AML) are commonly treated using a daunorubicin-cytarabine ±etoposide (DA/ADE) backbone as developed by the Medical Research Council (MRC) AML 12 trial and since adopted by the Children's Oncology Group (COG). This regimen is an anthracycline-intensive regimen, incorporating mitoxantrone in the consolidation phase, with a stated cumulative cardiotoxic exposure of ~444 mg/m 2 doxorubicin equivalents, though recent data suggests mitoxantrone is more cardiotoxic than previously considered (~10x more cardiotoxic than doxorubicin instead of 3x). The MRC AML15 trial randomized DA/ADE versus fludarabine-cytarabine-idarubicin (FLAG-Ida) and found superior event-free survival (EFS) from FLAG-Ida, with fewer cycles of intensive chemotherapy (4 vs 5), and less than half the cardiotoxic exposure, potentially reducing acute and long-term morbidity. Experience with FLAG-Ida as frontline therapy in children with de novo AML is limited. Following MRC 15, minimal residual disease (MRD) monitoring has become standard of care for risk stratification in AML, but MRD data for the FLAG-Ida regimen in children has not yet been reported.
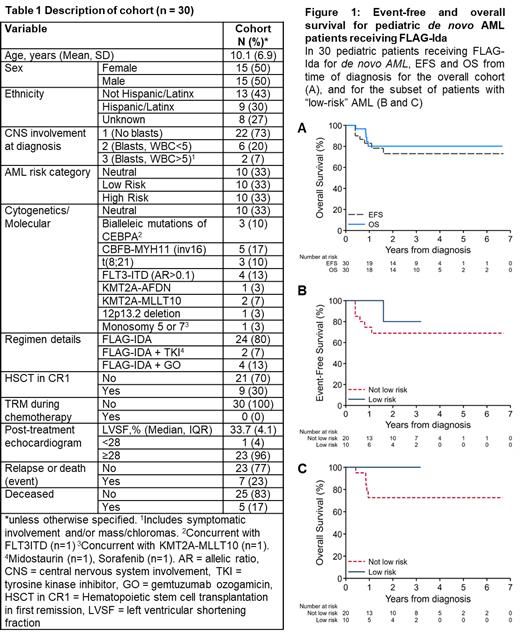
We collected data from 30 pediatric patients (age 0.3-20.0 years) with newly diagnosed de novo AML (no preceding myelodysplastic syndrome, secondary AML, or prior malignancy) and no underlying genetic disease (e.g., Trisomy 21, Fanconi Anemia, Kostmann Syndrome, Shwachman-Diamond Syndrome) treated at our institution with MRC 15-style FLAG-Ida between 2014 and 2021 (Table 1). Each case was characterized by cytogenetics, chromosomal microarray analysis (since 2015, in 29/30), and a proprietary molecular testing panel (since 2017, in 21/30); AML risk category was assigned at diagnosis using the contemporary COG classification (AAML1831). Following Induction I and II, MRD was measured in each patient by multiparameter flow cytometry using a 'difference from normal' approach (MRD+ defined by contemporary COG threshold, MRD≥0.05%). Patients with poor disease response (MRD+) or high-risk cytogenetics proceeded to hematopoietic stem cell transplantation (HSCT) in first remission. Patients routinely received antimicrobial prophylaxis with sulfamethoxazole-trimethoprim, an azole or echinocandin, and levofloxacin. The study was approved by the Institutional Review Board.
Following Induction I, 28/30 (93%) patients were MRD negative, and 30/30 (100%) patients following Induction II. Only 1/20 (5%) patients without high-risk AML required HSCT for slow-responding disease; 1/30 (3%) patients were unable to proceed to consolidation (prolonged myelosuppression). No patient developed treatment-related mortality (TRM) during pre-HSCT chemotherapy (2 subsequent to HSCT). Cardiac evaluation in surviving patients was normal (LVSF>28%) post-therapy in 23/24 (96%). Median survival for patients alive at last follow-up was 2.3 years with overall 3-year EFS and overall survival (OS) of 73±9% and 80±8%, respectively (Figure 1A). Survival for patients in the low-risk subset is depicted (Figure 1B, 1C); all low-risk patients were alive at last follow-up.
FLAG-Ida was well tolerated and effective in our pediatric AML population with no TRM, excellent early disease response by MRD, and encouraging albeit early EFS and OS. This compares favorably with the adult MRC AML 15 experience as well as with the COG DA/ADE regimen, particularly for MRD response (in AAML0531, ~30% patients were MRD+ following Induction I [Brodersen et al 2020]). These data support our continued use of FLAG-Ida in pediatric AML alone or in combination with targeted and/or molecular therapies. Further study of FLAG-Ida as frontline therapy in children with AML is warranted.
Gaynon: Takeda pharmaceuticals: Other: member of DSMC committee. Orgel: Jazz Pharmaceuticals: Consultancy.